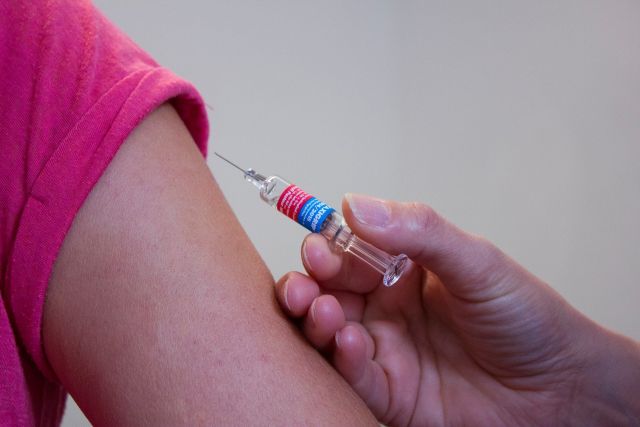
The Federal Biomedical Agency began clinical trials of its anticoid vaccine on July 19, according to the FMBA website.
The agency has received permission from the Russian Ministry of Health.
200 people between the ages of 18 and 60 will participate in the research.
Recall that the FMBA scientific center received on April 11 a patent for the drug Mir-19. Its name means small interfering RNA. The drug is expected to inhibit virus replication and prevent more severe forms of COVID-19.
Last week it was reported that the drug would be relevant to British and Indian strains of the coronavirus. & Nbsp;

